|
Research:
Synthesis of Biological Active Molecules
Our research is focus on the investigation of new approaches toward the
synthesis of biological active molecules, as briefly described below. If
you need further information, please do not hesitate to contact us:
luizfsjr @ iq.usp.br.
Present
Project:
Iodo and Iodine(III)
in the Synthesis of Biological Active Molecules
In the last years, hypervalent iodine
reagents have become an essential tool in Synthetic Organic Chemistry,
because of the great number of reactions that can be performed in excellent
yield and selectivity (regio-, diastereo-, andenantioselective). One of
these reactions is the oxidative rearrangement of olefins and ketones.
However, these rearrangements promoted by iodine(III) were explored only in
a superficial manner (See, for example, Silva Jr, Molecules 2006, 11, 421).
Considering our previous experience in the
study of oxidative rearrangements promoted by thallium(III), particularly
in ring contractions, we decided to explore iodine(III) reagents to promote
oxidative rearrangement that would lead to ring contraction or to ring
expansion of six-membered ring substrates.
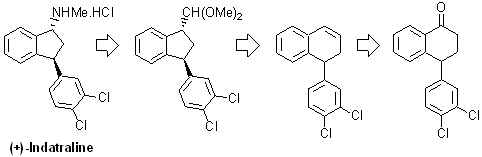
Using a ring contraction reaction as key step,
we plan to accomplish a diastereoselective synthesis of (+)-indatraline.
The abuse of drugs, such as cocaine, is a great problem to our society.
Considering our experience in the synthesis of indanes and the present
panorama related to the synthesis of 3-phenyl-1-indanamines, we plan to
investigate a new and general approach for the synthesis of indatraline and
its derivatives, which are a class of compounds that display several
interesting pharmacological and promising activities, such as in the treatment
of abuse of drugs, as cocaine and amphetamines.
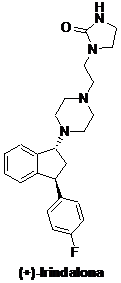
Irindalone is a trans-1-piperazine-3-phenyl-indan that has great affinity to
the receptors of serotonine2. This property makes this molecules
a promising anti-hipertensive, as reported by Bogeso and co-workers.
Studies toward the synthesis of (+)-irindalone are rare. Considering the
structural similarity between irindalone and indatraline, we plan to use a
similar approach to synthesize (±)-irindalone and (+)-irindalone. The
synthesis of other piperazines analogues, such as (+)-tefludazine, may be
investigated.
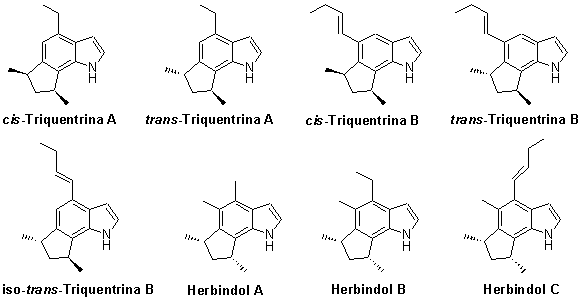
Studies
related to marine natural products have increased since the 90's, giving a
number of new structures. Among them, anti-cancer compounds were
discovered. In this context, we plan to synthesize some alkaloids isolated
from marine sponges, whose structures are shown below.
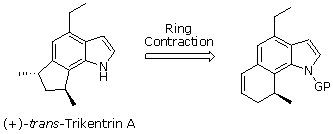
The strategy to achieve the asymmetric synthesis
of trans-trikentrin A will use a
ring contraction reaction in the key step, as shown below. Thus, a new and
general approach will be established to obtain natural or non-natural
bioactive poli-alkylated indols.
Other research
project are ongoing in our research group, such as i) ring expansion
reactions to obtain seven- and eight- membered rings; ii)
iodine(III)-catalyzed reactions; iii) investigation of the properties in
solution of hypervalent iodine reagents using ESI-MS; and iv) study of the
mechanism of iodine(III) oxidations using ESI-MS. If you have interest to
work in any of these projects, send us a message: luizfsjr@iq.usp.br.
|