Examples of radicals detected by the group.

-Professor of Biochemistry; Member of the
Brazilian Academy of Sciences, 2002; International EPR Society Medal, 2002; Order
of Scientific Merit, 2006; Scopus Prize, 2009; TWAS Member, 2011.
Laboratory of
Oxidants and Radical Biochemistry and EPR

Examples of radicals detected by the group.
|
-Professor of Biochemistry; Member of the
Brazilian Academy of Sciences, 2002; International EPR Society Medal, 2002; Order
of Scientific Merit, 2006; Scopus Prize, 2009; TWAS Member, 2011. |
RESEARCH INTERESTS
|
Our group has always been
focused in understanding the molecular mechanisms by which radicals and
oxidants mediate biological responses that range from signaling circuits
involved in physiology and pathophysiology to cellular and tissue injury. By
the use of electron paramagnetic resonance (EPR) and kinetics, among other
experimental approaches, we have contributed to the characterization of novel reactive species, such as alkyl/aryl radicals (R●/Ar●), peroxynitrite (ONOO-),
peroxymonocarbonate (HCO4-) and carbonate radical (CO3●-),
as well as of their biological sources and fates. By studying the reactivity of
radicals and oxidants with potential biological targets in vitro and in vivo,
we aim to continue contributing to advance the field of redox biology. We concur
with the view that further advances require interdisciplinary approaches
combining system biology with rigorous chemical and biological studies. |
Connecting the chemical and biological
properties of nitric oxide. From Toledo Jr and Augusto, Chem. Res. Toxicol. 25,
975, 2012. Copyright American Chemical Society. |
Openings for pos-docs and graduate students.
CURRENT RESEARCH
Presently, we are addressing the following
problems.
i) The redox
properties of the main physiological buffer, the pair bicarbonate/carbon
dioxide. In addition to contribute to the understanding and control of numerous
pathophysiological states and clinical conditions (for instance, emphysema, respiratory
muscle paralysis, pulmonary fibrosis), these studies may impact our view on how
increased levels of atmospheric carbon dioxide may affect life on Earth.
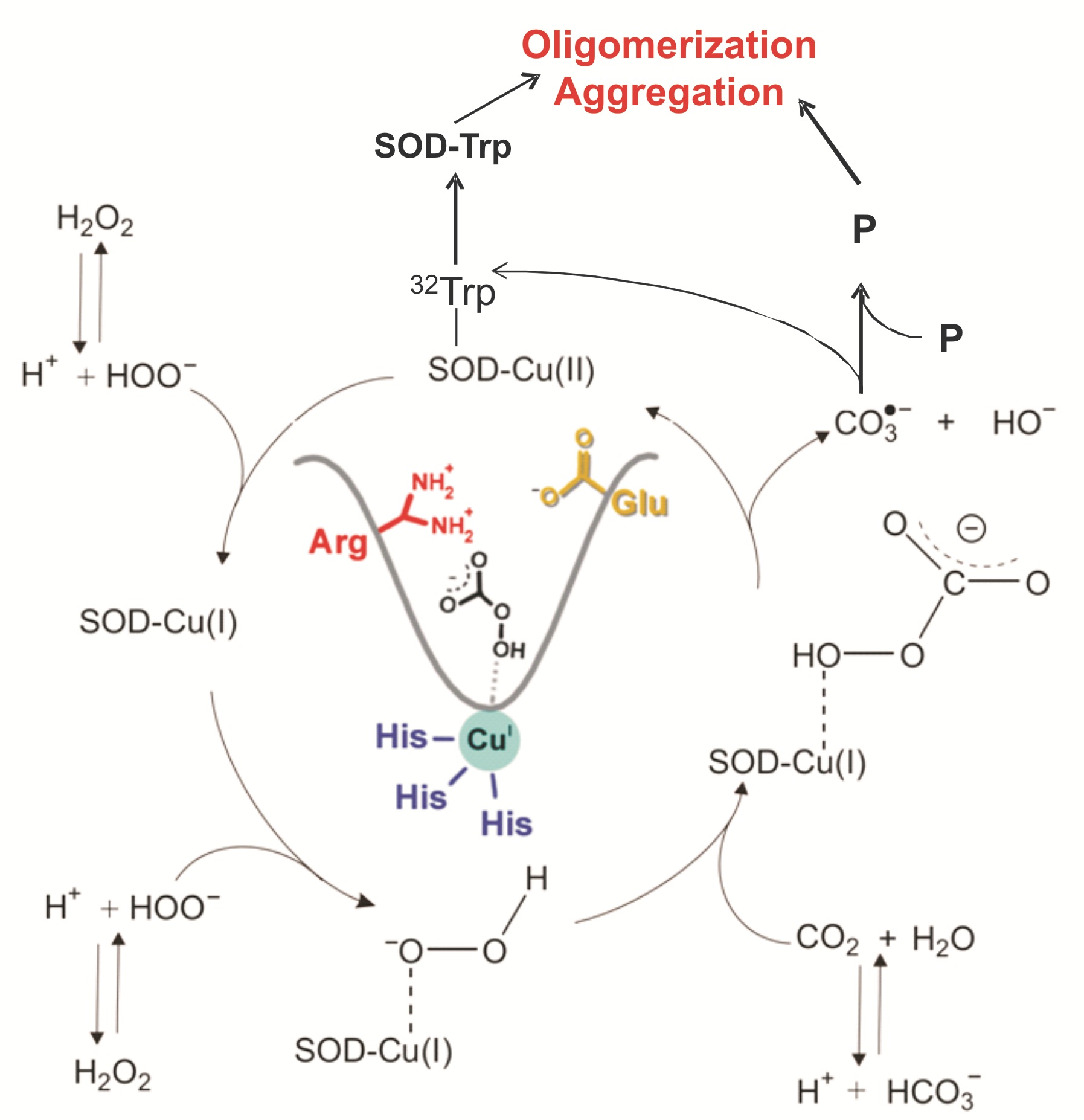 ii)
The structural and mechanistic properties of the human enzyme superoxide
dismutase1 that modulate its pro-oxidant and pro-aggregating properties. It is
expected that these studies will contribute to the elucidation of pathogenic
mechanisms underlying neurodegenerative diseases, in particular those involved
in amyotrophic lateral sclerosis.
ii)
The structural and mechanistic properties of the human enzyme superoxide
dismutase1 that modulate its pro-oxidant and pro-aggregating properties. It is
expected that these studies will contribute to the elucidation of pathogenic
mechanisms underlying neurodegenerative diseases, in particular those involved
in amyotrophic lateral sclerosis.
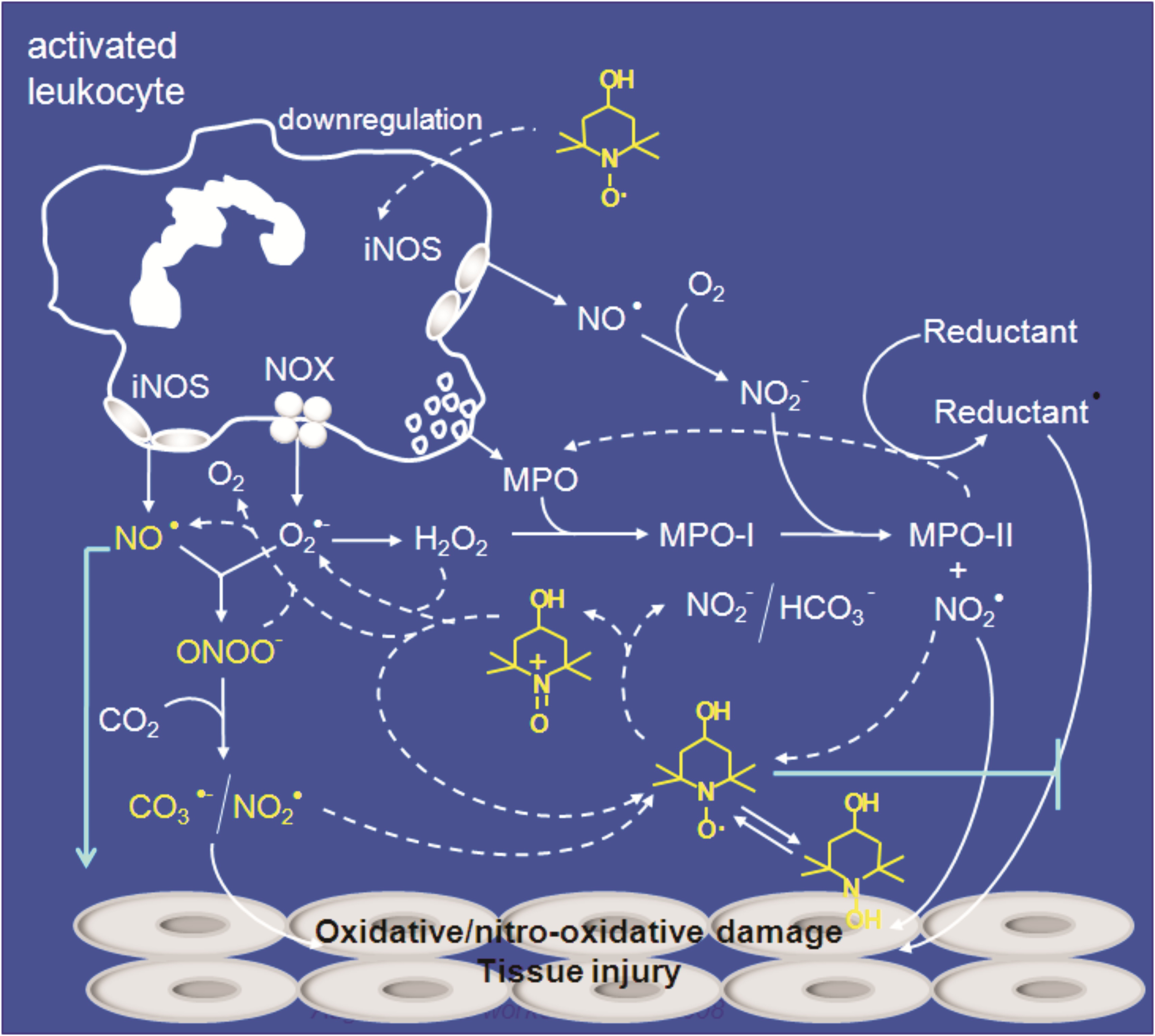
iii) The mechanisms
by which cyclic nitroxides are protective against oxidative and nitro-oxidative
damage in vitro and in experimental animals. In our view these studies are
relevant because nitroxides may provide new antioxidant and anti-inflammatory strategies. Mechanisms by
which the cyclic nitroxide tempol can attenuate inflammatory injury. From
Augusto et al, An Acd. Bras. Cienc, 2008; Vaz and Augusto, Proc. Natl Acad Sci
USA, 2009; Queiroz et al, Biochem. J. 2011.
iv) The kinetics of the reactions of proteins (heme
proteins, thiol proteins, etc) with oxidants and radicals. These studies are still
limited in the literature but are useful to unravel the physiological and pathophysiological
roles of species that are elusive under biological conditions as oxidants and
radicals are. (For a review see, Toledo
Jr e Augusto, Chem Res Toxicol 25, 975,
2012).
Openings for pos-docs and graduate students.
|

Copyright Oficina de Textos |
PUBLICATIONS Total publications (up to May 2012): -116 articles in peer-reviewed journals; -11 book chapters; -1 book (Portuguese). -h index= 34 (webofscience, May 2012).
|
Selected articles
Augusto, O.,
Bonini, M. G., Amanso, A. M., Linares, E., Santos, C. C. X. and de Menezes, S.
L. (2002) Nitrogen dioxide and
carbonate radical anion: two emerging radicals in Biology. Free Radic. Biol. Med. 32,
841-859.
Bonini, M. G., Radi, R.,
Ferrer-Sueta, G., da- Costa Ferreira, A. M. and Augusto, O. (1999) Direct
detection of the carbonate radical anion produced from peroxynitrite and carbon
dioxide. J. Biol. Chem. 274,
10802-1086.
Augusto, O., Beilan,
H. S. and Ortiz de Montellano, P. R.
(1982) The catalytic mechanism of cytochrome P450. Spin trapping evidence for
one-electron substrate oxidation. J.
Biol. Chem. 257, 11288-11295.
Augusto, O., Kunze, K. L. and Ortiz de Montellano, P. R.
(1982) N-phenyl protoporphyrin IX. Formation in the hemoglobin-phenylhydrazine
reaction: evidence for a protein-stabilized iron phenyl intermediate. J. Biol. Chem., 257, 6231-6241.
Quijano, C., Alvarez, B., Gatti, R. M., Augusto, O. and Radi, R. (1997)
Pathways of peroxynitrite oxidation of thiol groups. Biochem. J. 322, 167-173.
Augusto, O., Gatti, R.M. and Radi, R. (1994) Spin-trapping studies of
peroxynitrite decomposition and of 3-morpholinosydnomine N-ethylcarbamide
auto-oxidation. Arch. Biochem. Biophys. 310, 118-125.
Gatti, R. M., Radi, R. and Augusto, O. (1994) Peroxynitrite-mediated
oxidation of albumin to the protein thiyl free radical. FEBS Letters 348, 287-290.
Laurindo, F. R. M., Pedro, M. A., Barbeiro, H. V., Carvalho, M. H. C.,
Augusto, O. and da-Luz, P. (1994) Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism.
Circ. Res. 74, 700-709.
Santos, C. X. C., Anjos, E. I. and Augusto, O. (1999) Uric acid
oxidation by peroxynitrite: multiple reactions, free radical formation and
amplification of lipid oxidation. Arch. Biochem. Biophys. 372, 285-294.
Bonini, M. G. and Augusto, O. (2001) Carbon dioxide stimulates the
production of thiyl, sulfinyl, and dissulfide radical anion from thiol
oxidation by peroxynitrite. J.Biol. Chem. 276, 9749-9754.
FINANCIAL SUPPORT


|
|
 |
http://www2.iq.usp.br/redoxoma/ |